
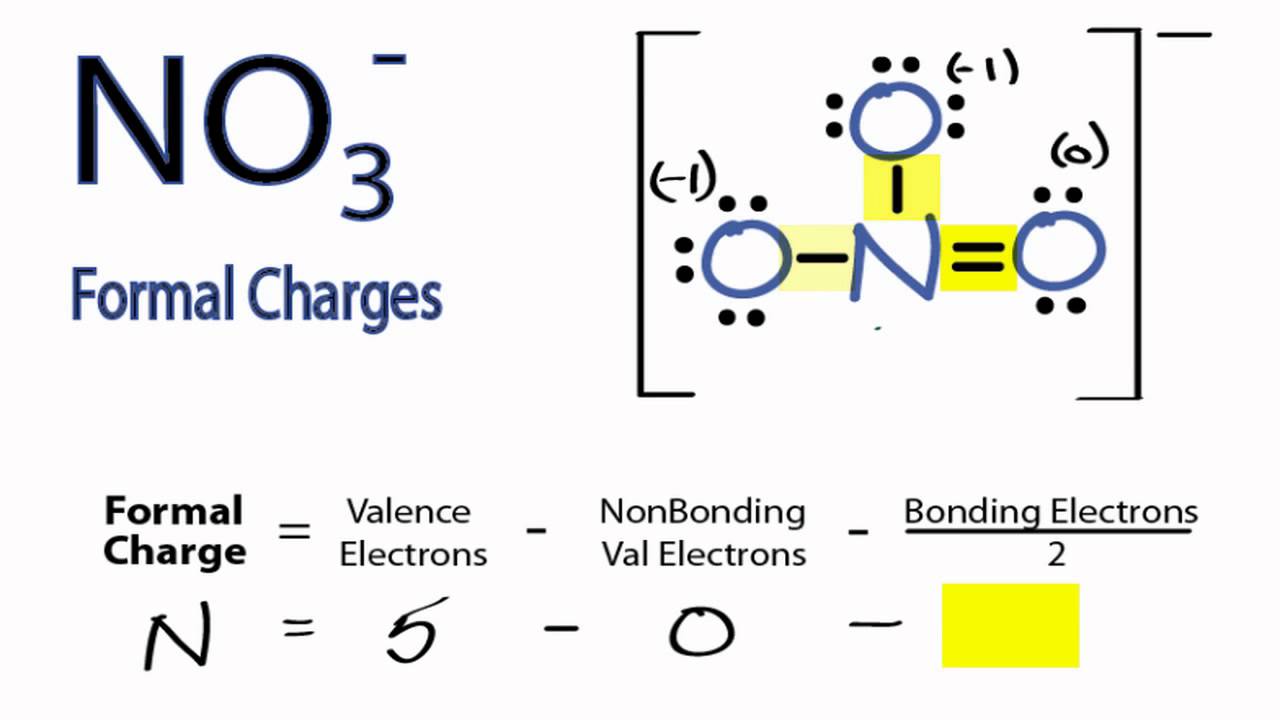
To obtain the formal charge of an atom, we start by counting the number of valence electrons for the neutral atom, and then subtract from it the number of electrons that it “ owns” ( i.e.
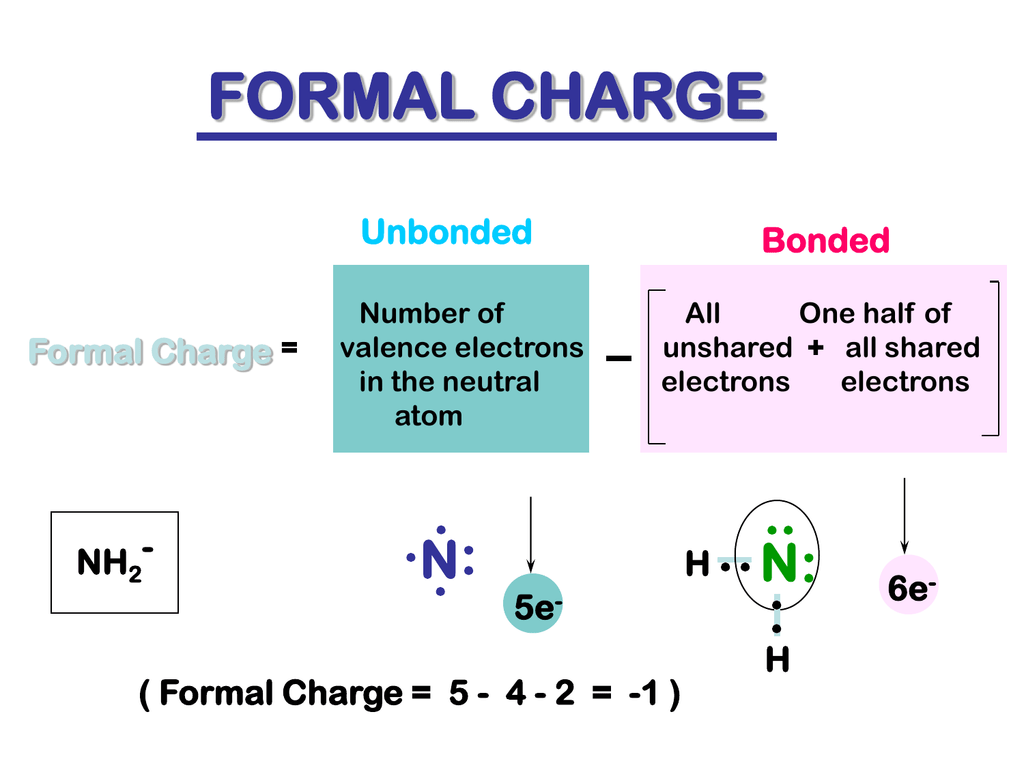
However, for brevity’s sake, there are many times when lone pairs and C-H bonds are not explicitly drawn out. The calculation is pretty straightforward if all the information is given to you. The formal charge FC is then calculated by subtracting NBE and B from VE. counting the number of bonds ( B) to the atom, or alternatively, counting the number of bonding electrons and dividing this by 2.Each lone pair counts as 2, and each unpaired electron counts as 1. counting the number of non-bonded valence electrons ( NBE) on the atom.(note: this is also equivalent to the effective nuclear charge Z eff, the number of protons that an electron in the valence orbital “sees” due to screening by inner-shell electrons.) 3 for boron, 4 for carbon, 5 for nitrogen, and so on).

evaluating the number of valence electrons ( VE) the neutral atom has (e.g.To calculate the formal charge of an atom, we start by:


 0 kommentar(er)
0 kommentar(er)
